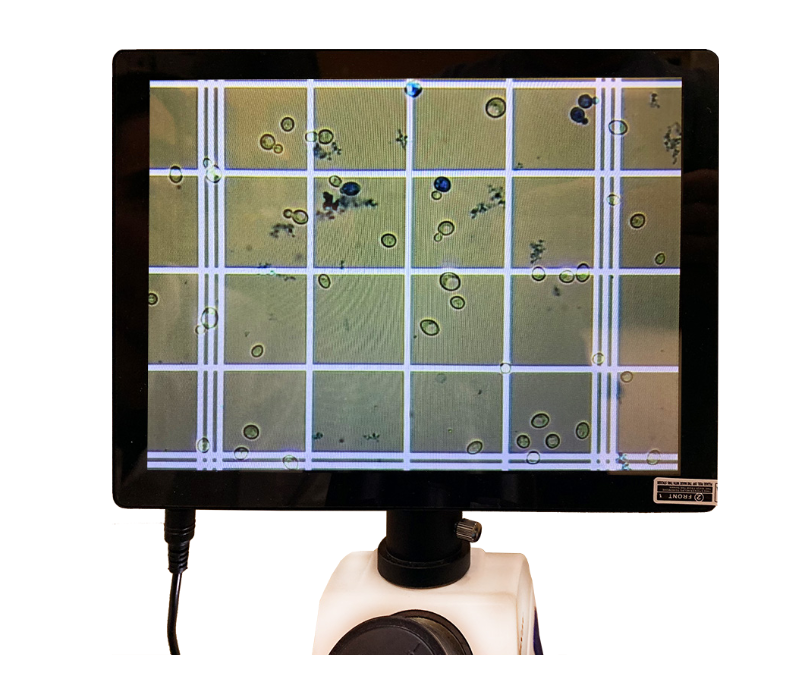
One of the most important factors in the quality of fermentation is the number of viable yeast cells in solution to ferment the sugar extracted from the mash into ethanol and CO2. This, in conjunction with the temperature of fermentation and health of the yeast population can be optimized to produce the flavor profile desired.
Equipment Needed:
- Microscope w/ 40x objective and 10x eye-piece (combined 400x magnification)
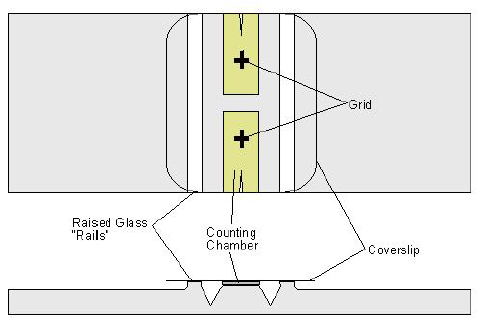
- Hemocytometer & cover slip
- Distilled water
- 5% H2SO4 for clumping if necessary
- Methylene blue or crystal violet for staining
- Pipette (Eppendorf with 1mL tip preferred, but a 1mL or 5mL glass pipette w/ a bulb works too)
- Clicker (a.k.a. hand counter)
Basic Procedure:
- Collect sample from yeast brink or fermenter
- Dilute to proper cell density
- Fill hemocytometer with dilute sample
- Count viable and dead cells
- Crunch a bunch of numbers to calculate how much slurry to add to the next fermentation
Note: The following two websites do a good job of stepping you through the above procedure in more detail. The most important points are in how the dilution is tracked and how the counting chamber is filled.
Getting into the Weeds:
Given that most of us don’t have the $25K or so that it takes to automate this process with a cellometer, we get to chase yeast cells around on a microscope, count them, and figure out which ones are alive. There are several pitfalls to counting yeast and pitching the correct amount to foster a healthy fermentation.
- Knowing how much yeast to pitch
- Accurate dilution tracking and measurement error
- Knowing which cells are viable for fermentation
- Mathematics of dilution and pitching rates
Determination of how much yeast you need:
A good rule of thumb for your pitch rate is 1 million cells per mL per degree Plato. This applies to ale wort under 16oPlato. For higher gravity wort, or for lagers, see below.
- If your ale wort has an original gravity of 1.050 (12.5oPlato), you should pitch 12.5 million cells per milliliter of wort. Note that 1 BBL = 117,335 mL
- (12.5 million cells / mL) * (117,335 mL/BBL) = 1.47 trillion cells / BBL of 12.5 oPlato wort
- For lagers, you should pitch 1.5 million cells per mL per oPlato
- (1.5 * 12.5 million) * (117,335) = 2.20 trillion cells / BBL of 12.5 oPlato lager wort
- For high gravity wort (above 16oPlato), add 0.5 million cells per mL per oPlato
- 23 trillion cells / BBL of 18 oPlato ale wort
Slurry Samples and Counting:
Now we need to get from trillions of yeast cells to liters of yeast slurry. This is where counting the yeast under a microscope comes in to determine the density of the yeast slurry (cells per unit volume).
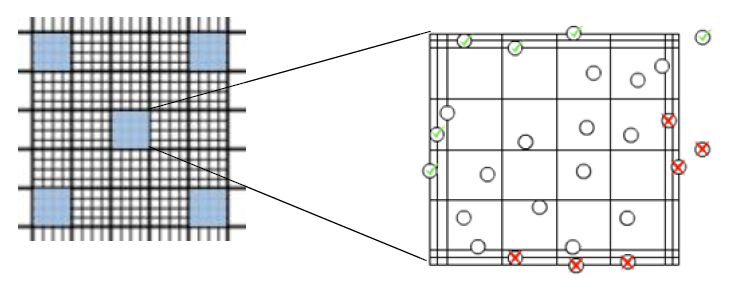
- The counting chamber on the hemocytometer is a grid with a fixed height
- The grid is etched into a 5×5 pattern of 25 squares, each containing a 4×4 pattern within
- You will typically count 5 of the 25 square 4×4 grids (see image below)
- The chamber has a volume of 0.0001 cm3
- Consistent methods to minimize variation are critically important
- Ensure that all samples and diluted solutions are representative and homogeneous
- Avoid double-counting cells as you move from square to square by only counting top and left sides of each 4×4 grid (do not count bottom and right sides)
- Track living and dead cells via staining with methylene blue (binds to dead cells) or crystal violet (binds to live cells)
- Track budding cells by only counting buds that are >50% the size of the mother cell
- The number of yeast cells you end up counting depends on the dilution rate
- Dilution with an accurate pipettor (i.e. Eppendorf) decreases error in the procedure
- A sequential dilution with a standard 1mL or 5mL bulb pipette and a few graduated cylinders is also a good option
- In the example calculation below, a 1-gram yeast sample is weighed and then diluted with 50mL of water. This dilute yeast solution is mixed 1:1 with a 0.01% methylene blue stain solution. The yeast slurry is assumed to have a density of 1.0 g/mL. This gives a dilution of 102x.
- Dilution with an accurate pipettor (i.e. Eppendorf) decreases error in the procedure
- Many breweries use kegs as yeast brinks. These are not usually transparent thus making it easier to measure by weight than by volume as you are moving yeast slurry in and out. A simple weight and volume measurement of a liter or two of your slurry will give you the density needed to pitch by weight.
Example calculations:
- (Cells x 5) x (dilution factor) x (1/0.0001cm3)
- (300 cells x 5 squares) x (102 dilution factor) x (10,000) = 153 x 107 cells/mL
- Dilution is (1:1 sample:stain (2x)) x (1mL sample in 50mL water (51x))
- You counted 300 cells in the hemocytometer. Out of these 300, you noted that 200 of them were alive to give 66.6% viability
- Now apply the dilution math above, and you come up with 1.53 billion cells / mL as a yeast slurry density
- Recall that you need 1.47 trillion cells per BBL for your 12.5 oPlato wort
- If you have 10BBL of wort:
- (10BBL) x (1.47 trillion cells needed per BBL) = 14.7 trillion cells needed
- (66.6% viability) * (1.53 billion cells / mL) = 1.02 billion viable cells per mL
- 7 trillion cells / 1.02 billion cells/mL = 14.37 liters of slurry needed
- (300 cells x 5 squares) x (102 dilution factor) x (10,000) = 153 x 107 cells/mL
Thoughts on Measurement Error:
- If you do a serial dilution of your yeast slurry, it is critical that the initial measurement of the mass or volume of yeast slurry be as accurate as possible.
- Measuring 1-gram of slurry on a scale with only 0.1-gram resolution is problematic. Your scale may read 1.0 gram, but you really don’t know if you have 0.91 or 1.09 grams of slurry, or anything between depending on the rounding protocols of the scale. This can introduce an error up to 10% in the amount of yeast with which you start.
- Similarly, if you are using a 10mL graduated cylinder for your first dilution, the position of the meniscus and the graduation scale itself can impact accuracy.
Written by Cy Bevenger Timnath Beerwerks