Brewing water can be viewed as the last frontier to many in our industry, but it needn’t be intimidating in the way that quantum mechanics or neuroscience are viewed by many. If you have control of your sanitation and yeast health, you can make good beer. The goal of this blog post is to give you a new tool in the endeavor of making great beer. By enabling you to make some easy adjustments to your water, you will improve the health of your brewing process from mash to draft.
Prerequisites
- Organic compounds and disinfectants can derail everything you do to make world class beer. The use of an activated carbon filter should take care of both of these if used properly. Some homebrewers use Campden tablets to remove chloramine instead of a carbon filter.
- Most city water reports don’t have the necessary information for brewing. You should send a sample to an analytical lab to get individual ion concentrations.
Definitions and Context in Brewing
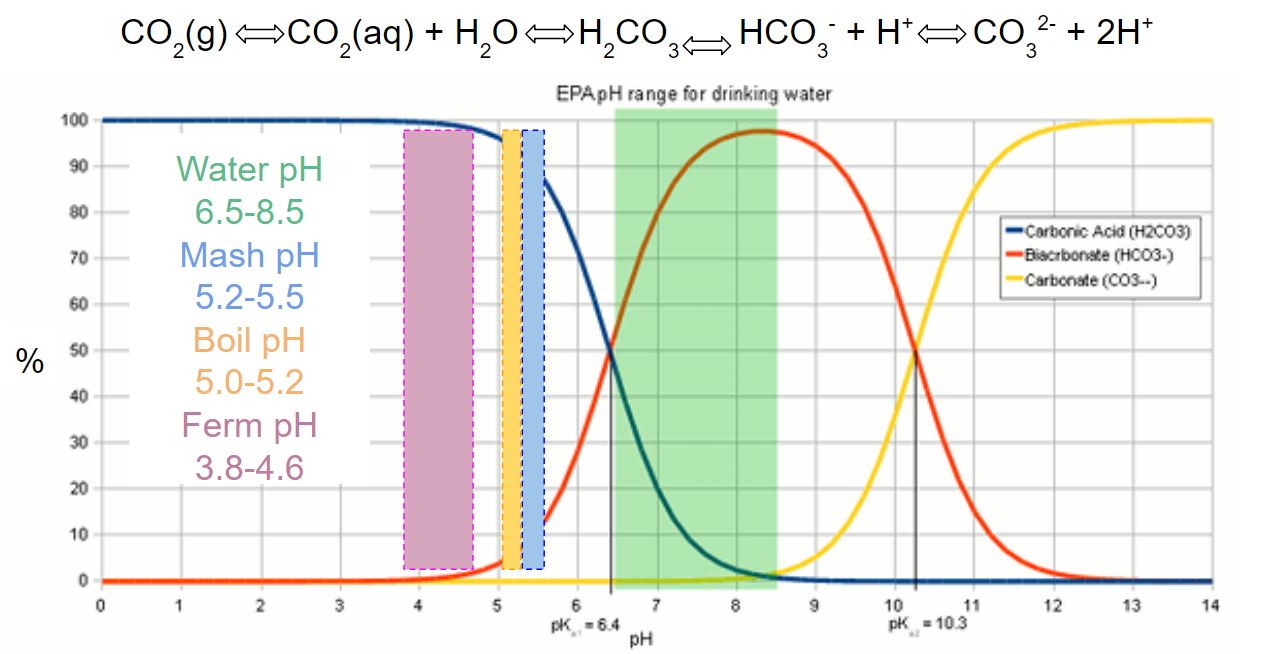
- pH: the measure of how acidic or basic a solution based on the concentration of H+ and OH– ions…the scale is logarithmic, so water with a pH of 5 has 10x the H+ ions as water with a pH of 6
- Mash pH between 5.2 and 5.5 necessary to prevent amylase enzymes from denaturing
- Low wort pH can negatively impact hot break, chill haze, hop utilization, etc…
- High wort pH in boil favors higher (and rougher) bitterness as well as higher Maillard reaction rates
- Fermentation pH below 4.4 favors diacetyl uptake, clarity, and biological stability
- Hardness: total concentration of multivalent metal ions in solution (Ca2+, Mg2+, etc…)
- Total Hardness: normalized expression of Ca2+ and Mg2+ concentrations expressed as ppm CaCO3
- Effective Hardness: a brewing term that ties Ca2+ and Mg2+ to residual alkalinity
- Strongly controlled by pH and temperature
- Alkalinity: the buffering capacity of water to resist a change in pH…roughly equal to the sum of carbonate and bicarbonate in solution
- Residual Alkalinity: a more specific way to determine how your brewing water will resist a change in pH…essentially, alkalinity minus hardness
- Higher residual alkalinity is generally better for darker styles due to the offsetting effects of malt (acidic) and water (basic)
- John Palmer has a great nomograph in How to Brew that will help you estimate residual alkalinity (http://howtobrew.com/book/section-3/understanding-the-mash-ph/residual-alkalinity-and-mash-ph)
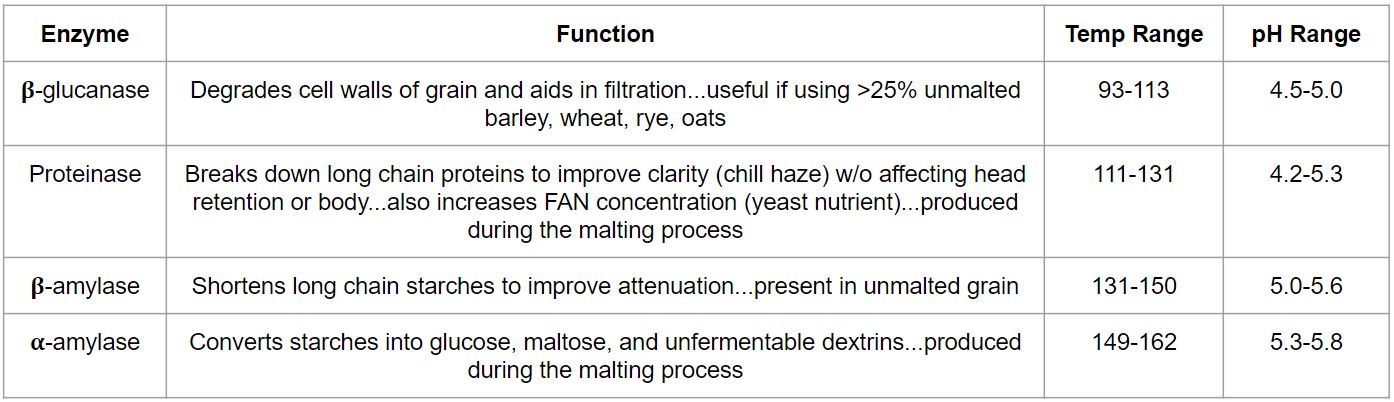
The role of enzymes
- Enzymes are the proteins in the mash that catalyze chemical reactions in the brewing process
- Historically, enzymatic activity was optimized to balance starch and sugar length coming out of the mash through the development of different mash schedules
- Properties including body, attenuation, and clarity were dialed in as a result
- Advancements in malting have simplified the mash schedules needed to make good beer
Water’s influence on classic styles
- In addition to the mash schedules developed in the historical brewing centers of the world, the chemical composition of each region’s water drove certain styles’ evolution
- Dublin – highly alkaline water allows for a healthy mash when acidic dark grains are used
- Pilsen – soft water with low alkalinity produces a healthier mash when only light grains are used…the low sulfate also enhances a mellow hop bitterness
- Burton – high calcium and sulfate enhance a clean hop bitterness
- Munich – high alkalinity from carbonates enhances smooth malty character
The role of minerals in process, flavor, mouthfeel, and balance
- Calcium helps control alkalinity via CaCO3 precipitation (lowers mash pH), aids in yeast flocculation, must be present for alpha amylase cleaving, helps temp stability of alpha amylase
- Recommended to be in the range of 50ppm to 200ppm
- High calcium may affect clarity in styles like Hefe and NEIPA (adding MgSO4 in place of CaSO4 may aid in maintaining hazy appearance)
- Magnesium generally comes from malt, but can be used to replace calcium if desired
- Not generally used in style discussions
- Typically in the range of 30ppm to 120ppm, and is an important yeast nutrient
- Mg2+ has been said to add richness to darker beers, but this is anecdotal
- MgSO4 can add a lingering, possibly sharp bitterness if used judiciously
- Balance of sulfate to chloride (SO42- : Cl–) strongly affects balance
- Sulfate enhances dryness and bitterness (ideally kept under 150ppm)
- Chloride enhances fullness and maltiness (ideally kept under 200ppm)
- The ratio of the two helps set the beer’s balance, but be wary of keeping calcium, sodium, and magnesium in check when making adjustments
- Many West Coast IPAs have a ratio of 2:1 sulfate to chloride
- Many New England IPAs have a ratio of 1:2 or 1:3 sulfate to chloride
- Sodium enhances sweetness, but can make a beer taste salty if overused
- Water softeners replace calcium and magnesium with sodium. This is generally not a good move in treating your water for brewing.
Putting Theory Into Practice: Two Extreme Examples (data is from BeerSmith)
- Denver’s water is reported as Ca=31.5ppm, Mg=8.5, Na=21.4, SO4=50.8, Cl=23.5, HCO3=104.0
- SO4:Cl ratio of 2.2, effective hardness of 28ppm, residual alkalinity of 58ppm
- Adjusting Denver’s water to Pilsen’s water requires the removal of ions either by reverse osmosis or distillation, or by dilution with distilled water
- Pilsen Ca=7.0ppm, Mg=2.0, Na=2.0, SO4=5.0, Cl=5.0, HCO3=15.0
- Blending 3 bbl of distilled water with 1 bbl of city water will yield 4 bbl of water within 10ppm of Pilsen’s water for all components
- SO4:Cl ratio of 2.2, effective hardness of 7ppm, residual alkalinity of 14ppm
- Adjusting Denver’s water to Dortmund’s water requires the addition of salts to increase ion concentrations…Dortmund’s hard water is great for strong, well-hopped, amber lagers
- Dortmund Ca=7.0ppm, Mg=2.0, Na=2.0, SO4=5.0, Cl=5.0, HCO3=15.0
- For each barrel (31 gallons) of water, adding 14.7g gypsum, 46.2g epsom salt, 16.7g calcium chloride, 31.2g baking soda, and 48.0g chalk will match Denver to Dortmund
- SO4:Cl ratio of 3.0, effective hardness of 216ppm, residual alkalinity of 227ppm
Written by Cy Bevenger, Timnath Beerwerks









